Does Spinach Has Vitamin C
Why Most Iron In Spinach Is Useless
Is Spinach Really the Superfood our Grandmothers Touted?
12th Feb 2016
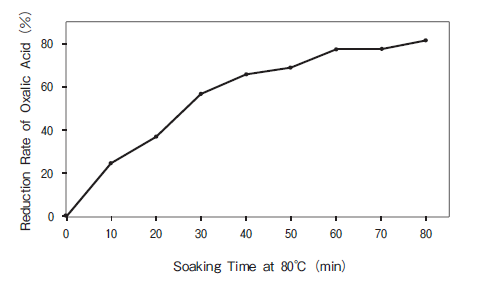
In order to claim that a food is a "source of" iron, a 100g portion must contain at least 15% of the recommended daily intake of iron (14mg)[1]. For a food to be classed as "high in" iron, it needs to have double this number. Compared to its leafy friends such as kale (1.7mg/100g), the iron content of spinach is high (between 2.1 and 2.7mg/100g)[2], however this falls well short of the 4.2mg/100g required to declare the food "high in iron". So, at best spinach is a "source of" iron and not "high in" iron. You have probably heard that a high proportion of iron in spinach has low bioavailability. This results in the majority of iron in spinach not being absorbed by the small intestine and therefore it is not usable by the body. Studies have shown that as little as 2% of iron from spinach is actually absorbed by the body[3]. This is quite low considering the average absorption of iron from meat is around 15 -35%[4]. So why is this? Firstly the form of iron found in spinach is non heme which is generally poorly absorbed in the gut compared to heme iron from animal sources. Heme iron is more resilient to changes in gastric pH and interactions from other dietary components so is more bioavailable. Secondly, spinach has such high levels of oxalic acid. This acid is naturally present in vegetables and binds with iron which blocks its absorption in the gut. Spinach has a higher level of oxalic compared to most vegetables with an approximate concentration of 1000mg/100g[5][7] .This is significantly higher than other vegetable such as kale (20mg/100g)[6], carrot (49mg/100g)[5], beetroot (67mg/100g)[5] and soybean (497mg/100g)[5]. Reduction of Oxalic Acid in Spinach There is much debate about whether it is possible to decrease the concentration of oxalic acid in various vegetables and how this is best achieved (mainly through cooking). A 2014 study found that soaking spinach at 80ºC for various periods of time can significantly reduce the concentration of oxalic acid. While soaking spinach for 80mins is pretty unrealistic for the average kitchen, a 20% reduction for 10 minutes seems achievable[6]. Another study found that boiling spinach for 12 – 15 minutes reduced the total soluble concentration of oxalic acid from 975mg to 477mg/100g[5]. In general boiling has been reported to reduce oxalates by 30% to 87%[7]. Both cooked and raw spinach have varying & plentiful nutritional benefits besides iron and are very low in calories. For example raw spinach is higher in vitamin C and cooked is higher in folate[2]. Eating a combination of cooked and raw spinach is the best way to ensure you are getting the best of both and keeps you from getting bored. To ensure maximum iron absorption, it's a good idea to avoid iron inhibitors such as phytic acid (which are high in grains and legumes), tannins, polyphenols (found in tea) and calcium when eating high non heme iron meals[8].
In terms of labelling? No
In terms of bio-availability? No
Don't give up on spinach just yet!
Combination Eating
Knowing what foods to eat and not eat with spinach can help maximise your iron absorption. Spinach should be eaten in combination with iron facilitators such as Vitamin C[8]. Eating non heme iron rich foods with heme iron rich foods such as meat can also help increase absorption[8].
Related articles
Source: https://www.nutritics.com/p/news_Why-Most-Iron-In-Spinach-Is-Useless

Tidak ada komentar: